– The success of the Phase II/III REVERSE-IT clinical study
The Phase II/III REVERSE-IT study is the last stage in the clinical development of TOTUM•63, before the submission of an application for a health claim recognized by the American, European and Canadian regulatory authorities. This international randomized, placebo-controlled, double-blind study called “REVERSE-IT” was launched in mid-2020. It enrolled 636 patients with prediabetes or early-stage untreated type 2 diabetes, in 52 centers from 7 countries.
The REVERSE-IT study has met its primary endpoint: TOTUM•63 reduced fasting blood glucose at 6 months versus placebo, on both a 3-intake/day and a 2-intake/day regimen.
Detailed results demonstrate TOTUM•63’s impressive efficacy on glucose metabolism, including plasma glycated hemoglobin (HbA1c), a biomarker established for microvascular risk, used for monitoring type 2 diabetes, with results comparable to some anti-diabetic drugs in a similar population.
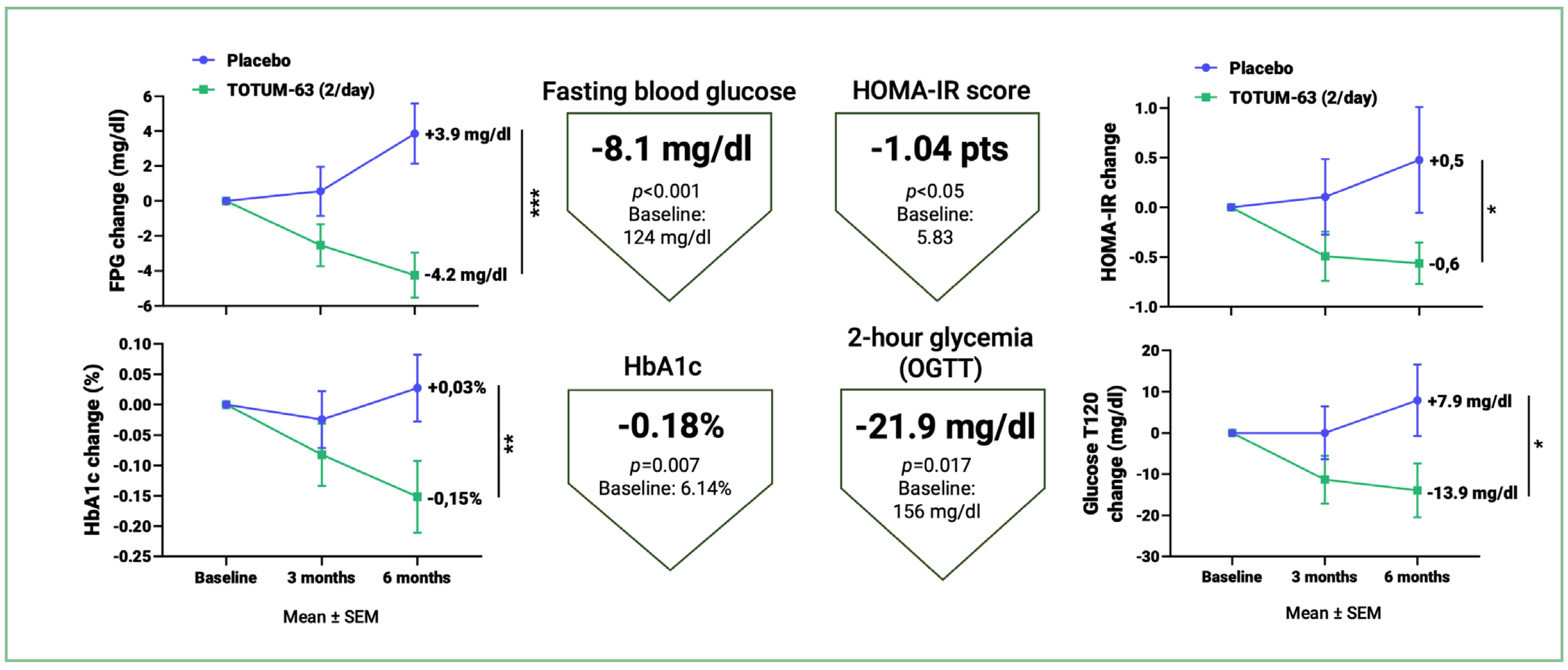
Among the main results, TOTUM•63 supplementation at 5 g/day, in 2 intakes, after
6 months:
- significantly reduces fasting blood glucose (-8.1 mg/dl), 2-hour blood glucose
(-21.9 mg/dl), glycated hemoglobin (-0.18%) and the HOMA-IR insulin resistance score (-1.04 pts), the main markers assessed in clinical practice, compared with placebo; - significantly reduces progression towards type 2 diabetes, with a 40% relative reduction in new cases of type 2 diabetes at the end of the study versus placebo;
- significantly reduces low-grade inflammation (-13% of patients above the threshold) involved in the pathogenesis of type 2 diabetes;
- demonstrates its efficacy on fasting blood glucose and glycated hemoglobin in early-stage untreated type 2 diabetics.
The study confirms TOTUM•63’s excellent safety profile, with no risk of hypoglycemia, very good tolerability, notably digestive, and a compliance exceeding 97%.
With such data, the REVERSE-IT study positions TOTUM•63 as an unparalleled non-drug innovation in the fight against the type 2 diabetes epidemic, benefiting the millions of people today facing the risks associated with this disabling chronic disease.