TOTUM•854 is a unique patented combination of plant extracts, specifically designed to reduce arterial blood pressure in people with mild to moderate elevated blood pressure, a risk factor for cardiovascular disease.
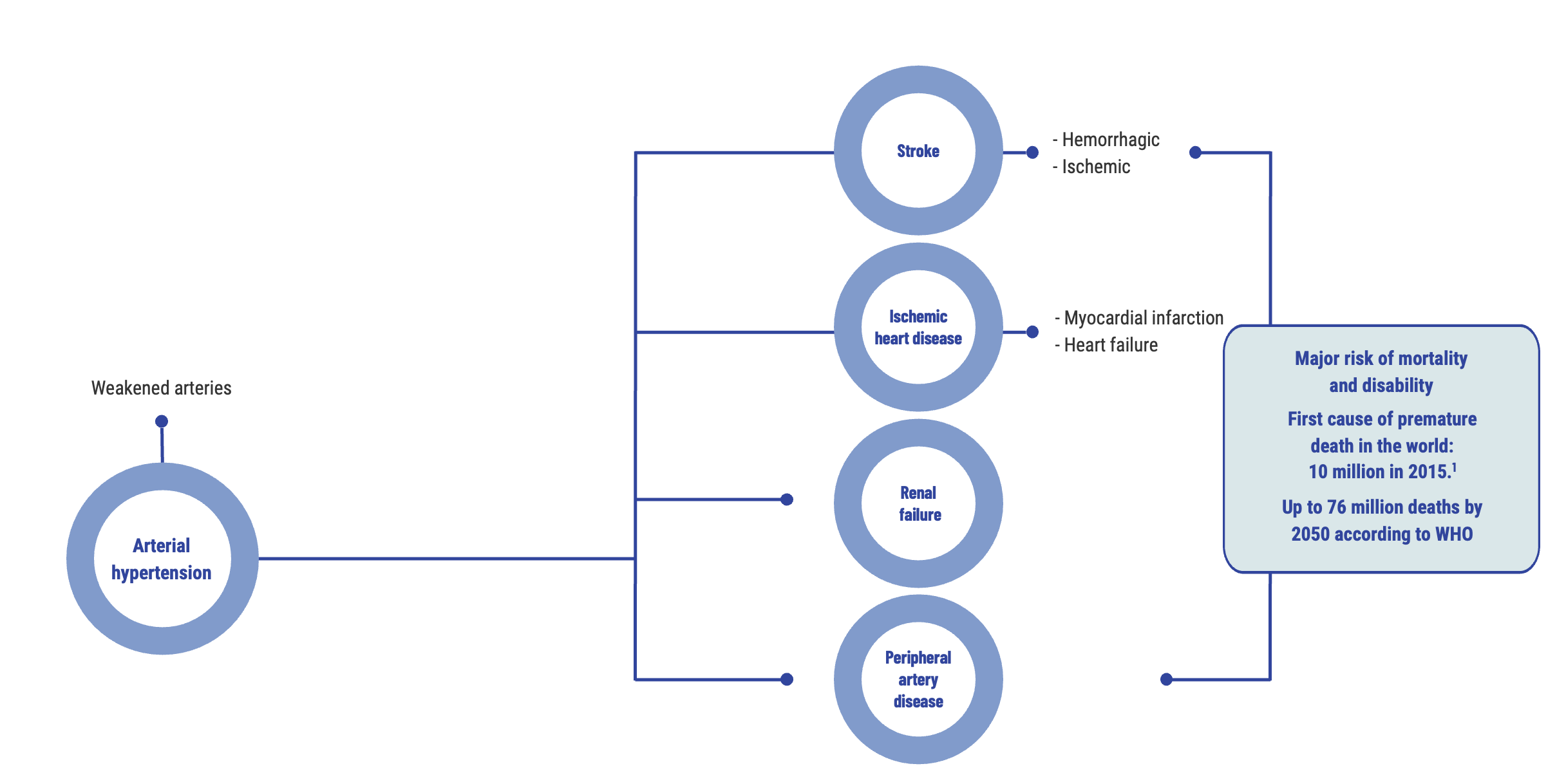
Arterial hypertension, the leading cardiovascular risk factor in the world
Arterial hypertension (AHT) is the world’s most prevalent chronic disease and the leading cause of premature death. It affects all countries, whether rich or poor¹. Over time, AHT weakens the arteries and promotes the onset of serious diseases, predominantly heart attacks, strokes, peripheral arterial diseases and kidney failure. It is the primary cardiovascular risk factor in the world.
Sources:
– Prise en charge de l’hypertension artérielle de l’adulte, Recommandation de bonne pratique, HAS, 2016 www.has-sante.fr/jcms/c_2059286/fr/prise-en-charge-de-l-hypertension-arterielle-de-l-adulte
¹ESC/ESH Guidelines for the management of arterial hypertension, European Heart Journal, 2018;
Key figures on arterial hypertension
1.3 billion people worldwide suffer from arterial hypertension.
Source: https://www.who.int/fr/news/item/19-09-2023-first-who-report-details-devastating-impact-of-hypertension-and-ways-to-stop-it
140/90 mmHg is the defined threshold for arterial hypertension in Europe. In the United States, the threshold value starts at 130/80 mmHg.
Source: 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension; 2017 ACC/AHA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults.
What is TOTUM•854?
TOTUM•854 is a unique patented combination of plant extracts, designed to reduce blood pressure in people with low to moderate elevated blood pressure, a risk factor for cardiovascular diseases.
TOTUM•854 could provide a new opportunity on a global market of more than one billion people, as no non-drug product has shown solid clinical evidence to date or received a health claim authorization relative to the reduction of blood pressure.
Illustrative purpose only – product not marketed
Preclinical results of TOTUM•854
Led in two different in vivo models, predictive of human physiology, the preclinical studies showed that TOTUM•854 prevented arterial hypertension. These preclincal data were obtained thanks to a partnership with the experimental unit of the Cardiovascular Pharm-Ecology Lab (LaPEC) of Avignon University.
These results were presented at annual congresses of the American Heart Association (AHA), the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH) in 2022.
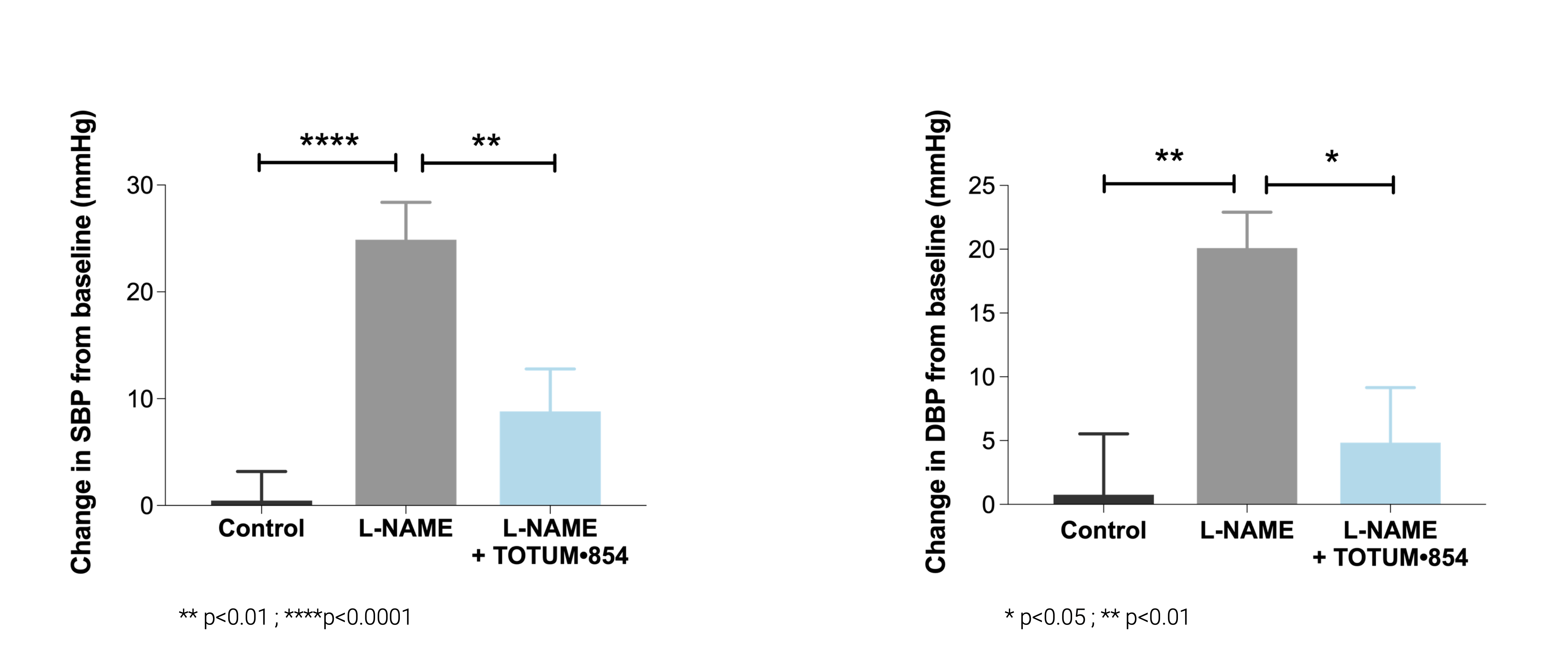
Hypertension model induced by L-NAME (NO synthase inhibitor)
In this classic model of hypertension, TOTUM•854 prevented the onset of hypertension, over 3 weeks, compared to L-NAME only. This significant preventive was shown on:
– the raise of dystolic blood pressure (SBP);
– the raise of diastolic blood pressure (DBP).
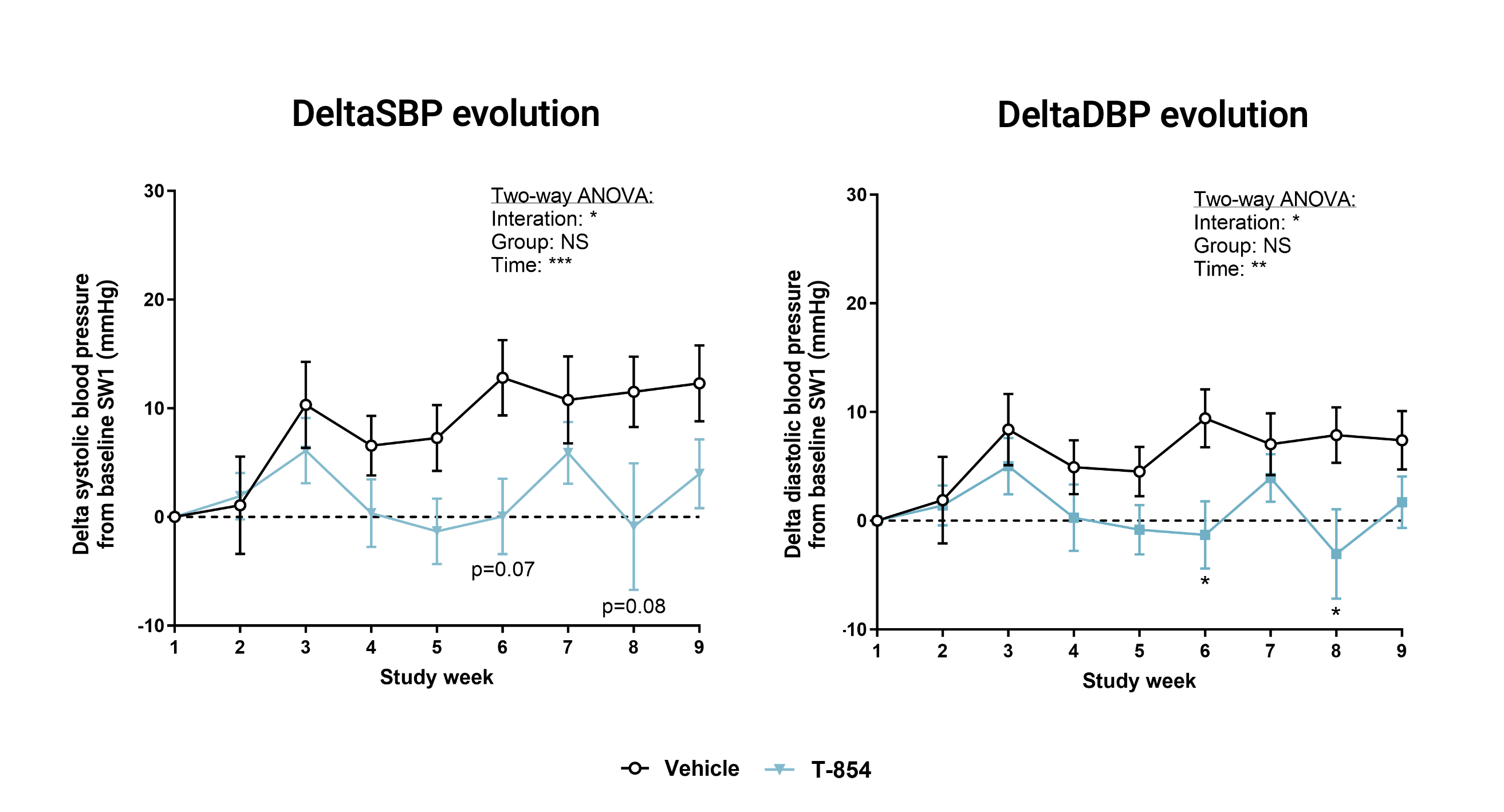
Polygenic hypertension model (SHR, Spontaneous Hypertensive Rat)
In this model of spontaneous hypertension, TOTUM•854 showed a positive effect, delaying the onset of arterial hypertension. An acute effect was also recorder after and oral intake of one dose of TOTUM•854.
TOTUM•854, a multitarget mode of action
The bioavailability and mode of action clinical study demonstrated a dual effect of TOTUM•854 on human endothelial cells of the vascular wall:
- The reduction of the angiotensin I-converting enzyme (ACE1) activity, one of the main modes of action known to reduce blood pressure.
- A protection of these vascular cells, particularly against inflammation and oxidative stress, which is key to preventing worsening of high blood pressure.
These results confirm the preclinical data, especially regarding the protection of the vascular wall.
Clinical development of TOTUM•854
The TOTUM•854 clinical development program includes three clinical studies, whose results will be required in Europe and the United States for health claim applications in the reduction of blood pressure, which is a risk factor for cardiovascular disease:
- 2 Phase II/III international and multicentric clinical studies, randomized, placebo-controlled and double-blind: INSIGHT and INSIGHT 2. These simultaneous studies will include 400 subjects each (800 in total) with low to moderate elevated blood pressure and will test two TOTUM•854 doses (3.7 g/day in INSIGHT, 2.6 g/day for INSIGHT 2). Their primary endpoint will be the reduction of systolic blood pressure. Recruitment of the first clinical Phase II/III (INSIGHT study) is completed.
- A MoA and bioavailability clinical study, to identify all TOTUM•854 metabolites and their effect on human cellular models, in 10 volunteers. Positive results were announced on January 30rd, 2023 (see “TOTUM•854, a multitarget mode of action“).
Intellectual property of TOTUM•854
Valbiotis has adopted a global strategy to protect its intellectual property: patent applications have been filed for TOTUM•854 in 63 countries, including key markets: Europe, USA, Canada, China, Russia, Japan, Brazil and Australia.
Patents have already been issued in 57 countries, including in the USA, Europe (39 countries), China, Russia or Japan, providing a very high level of protection.
